A 55-year-old man with no past medical history presented to an outside hospital with 1 week of progressive dyspnea, dry cough, and persistent substernal chest pain. He was transferred to our facility for further care, due to his imaging findings and lack of improvement after 5 days of vancomycin and piperacillin/tazobactam.
On presentation, he denied fever, chills, or other constitutional symptoms. There was no history of illicit drug use, foreign travel, tuberculosis, or occupational exposure. He was ill appearing, and had a respiratory rate of 25 breaths/min, blood pressure of 130/85 mm Hg, and a heart rate of 125 beats/min. His oxygen saturation was 98% on 2 L/min oxygen delivered via a nasal cannula. His cardiorespiratory examination was unremarkable, with no stridor, wheezing, or crackles heard on auscultation. The remainder of the physical examination was normal.
Laboratory data, including tests for connective tissue diseases with anti-nuclear antibodies and anti-neutrophil cytoplasmic antibody, was unremarkable, excepting a mild leukocytosis with neutrophilia. Sputum and blood cultures were obtained. He was continued on broad-spectrum antibiotic coverage. Chest computed tomography (CT) revealed a 3-cm segment of circumferential thickening of the trachea from the thoracic inlet to the T4 level (Figure 1) with other aspects of the lungs and heart being normal. The next day, the patient required endotracheal intubation for worsening respiratory distress. Flexible bronchoscopy revealed a 2.5-cm segment of tracheal necrosis limited to the anterior and lateral walls (Figure 2).
Figure 1.
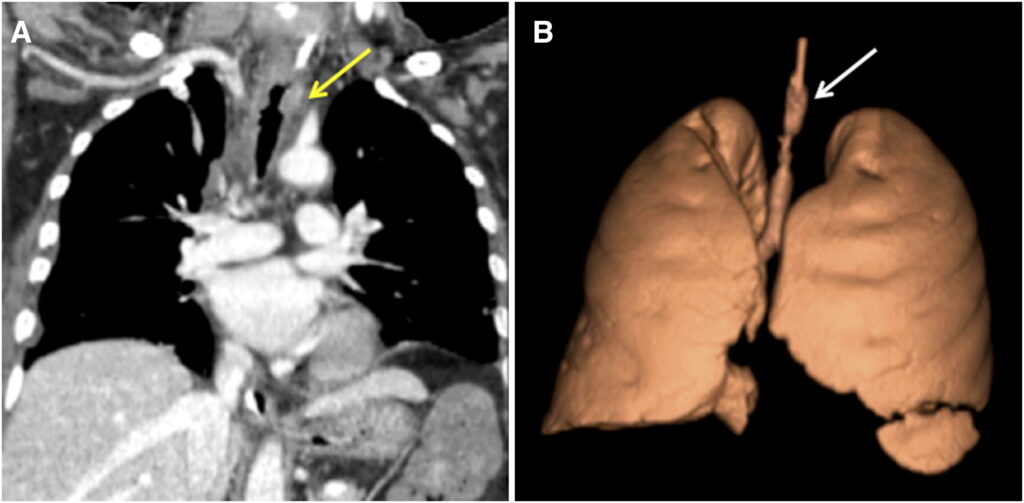
(A) Computed tomography (CT) chest coronal section demonstrating circumferential irregular thickening/nodularity of a segment of the trachea (yellow arrow). (B) Three-dimensional reconstruction of the CT showing the same irregular circumferential area of tracheal thickening (white arrow).
Figure 2.
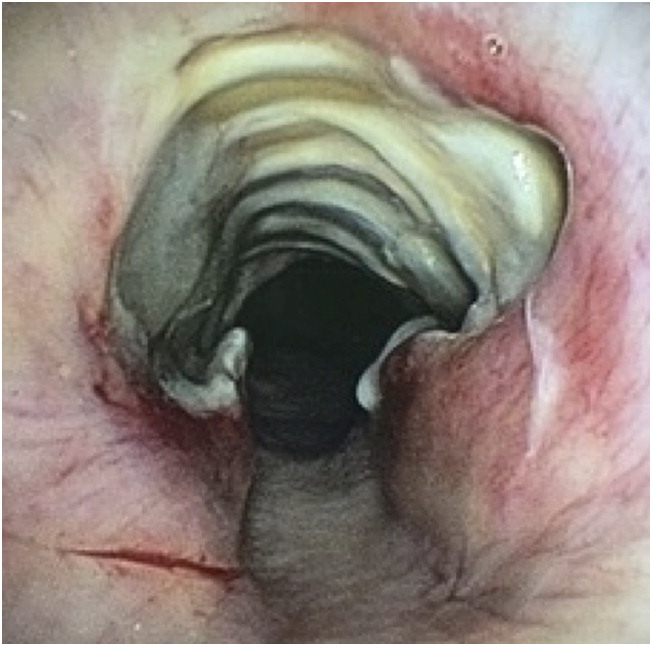
Bronchoscopic view of a segment of necrotic tracheal mucosa involving the anterior and lateral walls, with sparing of the posterior membranous portion.
Clinical Reasoning, Diagnosis, and Management
Several endobronchial (EB) biopsies were taken from the tracheal mucosa adjacent to the necrotic areas. The differential diagnosis at this point was broad (Table 1). Although hematogenous metastases to the trachea can occur, most cancers seen in the trachea are from extension and invasion from adjacent structures. Primary tracheal cancers are very rare, but the possibility of a tracheal adenoid cystic cancer, squamous cell cancer, or a primary mucoepidermoid cancer could not be excluded without histopathology. Granulomatosis with polyangiitis often involves the subglottic tracheal region with stenosis, ulceration, calcifications, or inflammatory pseudopolyps. These lesions may progress to tracheal necrosis and deformation, and may involve the posterior tracheal membrane. Other tracheal diseases, including relapsing polychondritis, can cause cartilaginous thickening and destruction that can lead to tracheomalacia. Ulcerative colitis can affect the tracheobronchial mucosa with ulcerations and irregular luminal narrowing. A small percentage of patients with sarcoidosis have granulomatous infiltration of the trachea or bronchi, some with the classic cobblestone appearance. Amyloidosis rarely causes multifocal tracheal plaques, and can cause stenosis.
Although the appearance of our patient’s tracheal involvement was not typical for rhinoscleroma, Klebsiella and other bacteria, including Staphylococcus aureus, were not completely excluded by the lack of improvement on antibiotics. In evaluation, QuantiFERON-TB Gold (Quest Diagnostics, Los Angeles, CA) and HIV testing were normal. Serum angiotensin converting enzyme and calcium levels were normal. Urinalysis with microscopy, and serum and urine protein electrophoresis, were also unremarkable.
This presentation was highly suspicious for a fungal tracheitis from invasive aspergillosis or mucormycosis, although the lack of immune compromise in our patient would make these diagnoses surprising. Coccidioides IgM and IgG antibodies (by enzyme immunoassay), Histoplasma urine antigen, serum galactomannan, and β-D glucan were negative. The tracheal mucosal biopsies revealed fungal elements suggestive of mucormycosis (Figure 3).
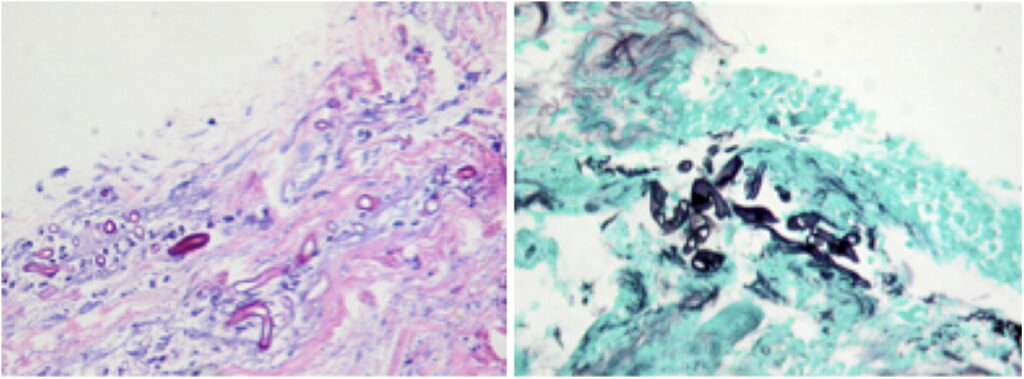
Figure 3.
Hematoxylin and eosin and Gomori methenamine silver stain of a tracheal biopsy sample showing broad, nonseptate hyphal fungal elements suggestive of Mucor. Magnification, ×40.
Table 1. Differential diagnosis
| Primary tracheal neoplasms, including adenoid cystic, squamous cell, or mucoepidermoid carcinoma |
| Granulomatosis with polyangiitis |
| Relapsing polychondritis |
| Ulcerative colitis |
| Sarcoidosis |
| Amyloidosis |
| Bacterial tracheitis due to Klebsiella rhinoscleromatis or Staphylococccus aureus |
| Fungal tracheitis from aspergillosis or mucormycosis |
The patient was newly diagnosed with diabetes mellitus, with a hemoglobin A1c of 9.5%. CT imaging and endoscopic evaluation demonstrated no evidence of rhinocerebral disease. The patient was diagnosed with isolated tracheal mucormycosis.
He underwent tracheostomy placement and parenteral therapy with amphotericin B and micafungin. Based on the extensive nature of a potential surgical intervention, and on the patient’s preference, we decided against surgery, and chose to assess our patient’s response to medical therapy with serial bronchoscopies, while having his airway protected with a Shiley XLT tracheostomy tube (Covidien [Medtronic], Woodland Hills, CA). Tight glucose control was maintained. After 4 weeks of combination parenteral antifungal therapy, a repeat bronchoscopy revealed complete healing of the previously necrotic tracheal mucosa (Figure 4).
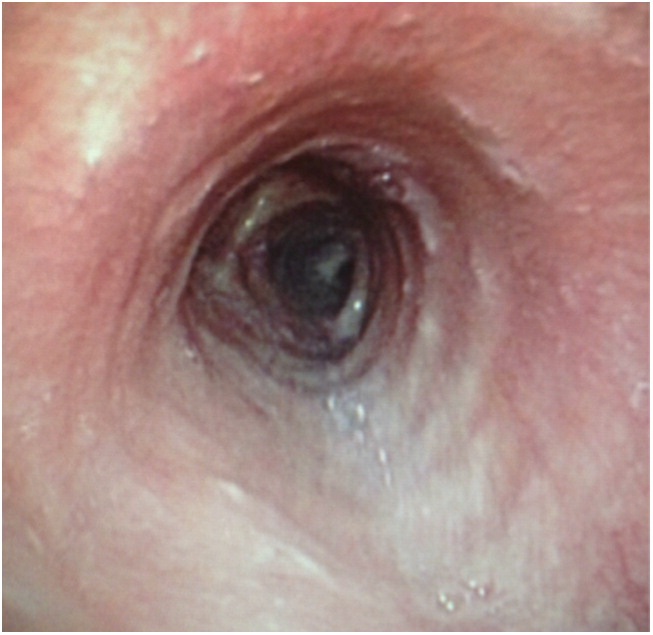
Figure 4.
Bronchoscopic view of the completely healed tracheal mucosa after 4 weeks of combination antifungal therapy.
Discussion
The most common route for fungi to enter the human body is inhalational. Most reports of fungal tracheobronchitis are caused by Aspergillus (1). In general, manifestations range from single or multiple discrete airway wall plaques to circumferential pseudomembranes of necrotic tissue, exudate, and hyphae (2). Mucormycoses are opportunistic infections caused by fungi in the class of Zygomycetes, found in the soil and in decaying matter. They most commonly cause rhinocerebral or pulmonary disease. Contiguous or concomitant EB involvement is infrequently reported, though probably underdiagnosed (3). EB mucormycosis usually presents as an obstructive mass or a grayish-white fibrinous plug, although ulcers, stenosis, pseudomembranes, and granular lesions have been reported (1). The extensive tracheal necrosis seen in our patient was likely a manifestation of its angioinvasive nature. Thus, with vascular invasion and the resulting thrombosis, tissue infarction is a disease hallmark. Hemoptysis is a common clinical symptom and a frequent cause of death in pulmonary mucormycosis.
Common predisposing factors for mucormycosis includes immune compromise due to corticosteroids, organ transplantation, hematological malignancies, or acquired immunodeficiency syndromes (4). Uncontrolled diabetes mellitus is a common risk factor for mucormycosis and the likely cause in our patient. Individuals with diabetes have increased serum iron because of impaired transferrin binding, which may contribute to a higher risk of fungal infections, due to increased fungal iron uptake that stimulates growth. Mucormycosis also has been reported in patients who are on deferoxamine therapy, but have no other risk factors (5). The hypothesis to explain why this iron chelator paradoxically increases susceptibility to mucormycosis is that the fungi use it as a siderophore. Local airway wall damage with neoplastic infiltration, nonfungal infection, or prolonged mechanical ventilation may also predispose to fungal colonization and invasion (2).
Mucormycosis can develop throughout the upper and lower airways. Rhinocerebral disease and pulmonary parenchymal involvement are more common than isolated tracheobronchial involvement (4). Interestingly, patients with diabetes mellitus have an apparent predilection for the development of EB lesions, accounting for a large number of reported cases in the literature (3).
The diagnosis of EB fungal disease requires a high index of suspicion, and largely depends on the histopathological demonstration of tissue invasion and growth on culture (1, 3, 4).
Biopsies of synchronous parenchymal or upper respiratory lesions may also be useful in the right clinical scenario, and often the clinical scenario may itself suffice to make a presumptive diagnosis, even in the absence of histopathological confirmation. Blood cultures rarely grow Mucorales species, despite their angioinvasive nature (3). Mucor species stain poorly with the periodic acid–Schiff and gram stains, but stain very well with Gomori methenamine-silver (6). They occur as broad (10 to 20 μm in diameter), nonseptate hyphae with branches at right angles (7).
The treatment of this deadly disease relies on early identification and timely institution of therapy, and is usually surgical in combination with amphotericin. Amphotericin B deoxycholate and its lipid formulations are the keystone of therapy. Although mucormycosis is traditionally thought to be resistant to azoles and echinocandins based on in vitro susceptibility studies, some retrospective data suggest that adding an echinocandin to a polyene regimen may improve outcomes (8, 9). With a reported mortality of approximately 50% (1, 4, 10), it is important to note one retrospective study in which 11 of 13 patients who also had a surgical intervention survived their disease (1). Correction of the underlying predisposing condition, such as diabetic ketoacidosis, is also important (3, 10).
We chose to assess our patient’s response to medical therapy with serial bronchoscopies and CT scans, while having his airway protected with a distal tracheostomy tube. Although combination antifungal therapy appears promising, its superiority to monotherapy still requires confirmation by randomized, controlled trials (8, 11).
Answers
1. What is the best way to establish the diagnosis?
Bronchoscopic tracheal mucosal biopsy.
2. What do you think is the diagnosis and what are the risk factors for this condition?
Fungal tracheobronchitis. Risk factors include immune compromise due to corticosteroids, organ transplantation, hematological malignancies, acquired immunodeficiency syndromes, and diabetes mellitus.
3. How would you manage this patient?
Antifungal therapy with serial bronchoscopic and imaging-based surveillance, with or without surgical intervention.
Follow-Up
Our patient was discharged home after 1 month with a Montgomery tracheal T-tube (Boston Medical, Los Angeles, CA) to help maintain an adequate airway. He was treated as an outpatient with isavuconazole, a recently approved azole with comparable efficacy, favorable pharmacokinetics, and better side-effect profile than amphotericin B (12).
At the 1-month clinic visit, his treatment course with isavuconazole was extended to 6 months based on persistent tracheal thickening on an interval follow-up CT scan. At the 6-month follow-up visit, his T-tube was removed and isavuconazole stopped, as he continued to do well. He has had no recurrence of symptoms at 1 year since his discharge, and successfully maintains tight glucose control with a glargine-based insulin regimen.
Insights
• EB Mucor usually presents as an obstructive mass or a grayish-white fibrinous plug, although mucosal necrosis can occur due to its angioinvasive nature.
• Prompt diagnosis, early initiation of antifungal therapy, reversal of host risk factors, and a case-based approach to surgery offer the best chances for survival of patients with mucormycosis.
• Although combination antifungal therapy is sometimes used for an initial intensive regimen, randomized trials have not validated this approach.
References
| 1 . | Karnak D, Avery RK, Gildea TR, Sahoo D, Mehta AC. Endobronchial fungal disease: an under-recognized entity. Respiration 2007;74:88–104.
Crossref, Medline, Google Scholar
|
| 2 . | Clarke A, Skelton J, Fraser RS. Fungal tracheobronchitis: report of 9 cases and review of the literature. Medicine (Baltimore) 1991;70:1–14.
Crossref, Medline, Google Scholar
|
| 3 . | Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med 2011;32:693–702.
Crossref, Medline, Google Scholar
|
| 4 . | Lee FY, Mossad SB, Adal KA. Pulmonary mucormycosis: the last 30 years. Arch Intern Med 1999;159:1301–1309.
Crossref, Medline, Google Scholar
|
| 5 . | Boelaert JR, Fenves AZ, Coburn JW. Deferoxamine therapy and mucormycosis in dialysis patients: report of an international registry. Am J Kidney Dis 1991;18:660–667.
Crossref, Medline, Google Scholar
|
| 6 . | Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev 2000;13:236–301.
Crossref, Medline, Google Scholar
|
| 7 . | Frater JL, Hall GS, Procop GW. Histologic features of zygomycosis: emphasis on perineural invasion and fungal morphology. Arch Pathol Lab Med 2001;125:375–378.
Crossref, Medline, Google Scholar
|
| 8 . | Reed C, Bryant R, Ibrahim AS, Edwards J Jr, Filler SG, Goldberg R, Spellberg B. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis 2008;47:364–371.
Crossref, Medline, Google Scholar
|
| 9 . | Spellberg B, Fu Y, Edwards JE Jr, Ibrahim AS. Combination therapy with amphotericin B lipid complex and caspofungin acetate of disseminated zygomycosis in diabetic ketoacidotic mice. Antimicrob Agents Chemother 2005;49:830–832.
Crossref, Medline, Google Scholar
|
| 10 . | Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005;41:634–653.
Crossref, Medline, Google Scholar
|
| 11 . | Spellberg B, Ibrahim A, Roilides E, Lewis RE, Lortholary O, Petrikkos G, Kontoyiannis DP, Walsh TJ. Combination therapy for mucormycosis: why, what, and how? Clin Infect Dis 2012;54:S73–S78.
Crossref, Medline, Google Scholar
|
| 12 . | Donnelley MA, Zhu ES, Thompson GR III. Isavuconazole in the treatment of invasive aspergillosis and mucormycosis infections. Infect Drug Resist 2016;9:79–86.
Medline, Google Scholar
|